Northwestern University research introduces a cost-effective catalyst made from molybdenum and table sugar that converts CO2 to carbon monoxide, presenting a viable method for transforming captured carbon into useful products such as fuel precursors.
The new catalyst could provide a potential solution for using captured carbon.
A new catalyst made from a cheap, abundant metal and ordinary table sugar has the power to destroy carbon dioxide (CO2) gas.
In a new Northwestern University study, the catalyst successfully converted CO2 into carbon monoxide (CO), an important building block for the production of various useful chemicals. When the reaction takes place in the presence of hydrogen, for example CO2 and to transform the hydrogen into synthesis gas (or syngas), an extremely valuable precursor for the production of fuels that can potentially replace gasoline.
With recent advances in carbon capture technologies, post-combustion carbon capture is becoming a plausible option to address the global climate change crisis. But how to deal with sequestered carbon remains an open-ended question. The new catalyst could potentially provide a solution to disposing of the potent greenhouse gas by turning it into a more valuable product.
The study will be published in the May 3 issue of the journal Science.
“Even if we stop emitting CO2 now our atmosphere will still have excess CO2 as a result of industrial activities of past centuries,” said Northwestern’s Milad Koshoei, who led the study. “There is no single solution to this problem. We need to reduce CO2 emissions and find new ways to reduce CO2 concentration already in the atmosphere. We have to take advantage of all possible solutions.”
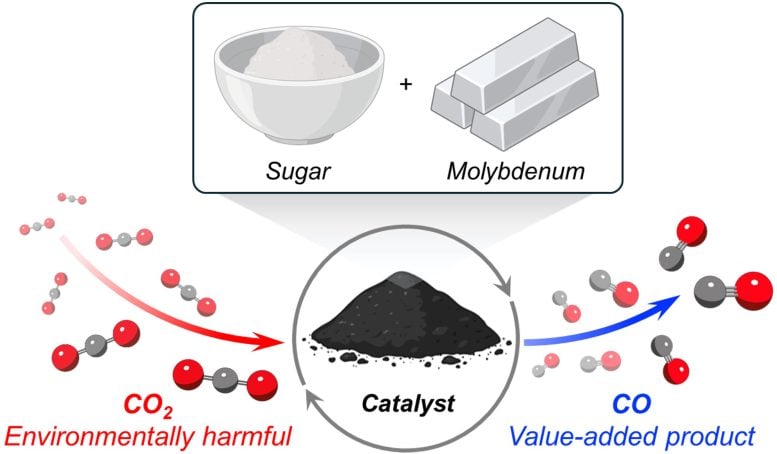
This schematic shows the complete process of creating the catalyst and using it to convert carbon dioxide. Credit: Milad Khoshooei
“We are not the first research group to convert CO2 in another product,” said Omar K. Farha of Northwestern, senior author of the study. “However, for the process to be truly practical, it requires a catalyst that meets several key criteria: affordability, stability, ease of production, and scalability.” Balancing these four elements is key. Fortunately, our material exceeds these requirements.”
An expert in carbon capture technologies, Farha is the Charles E. and Emma H. Morrison Professor of Chemistry in Northwestern’s Weinberg College of Arts and Sciences. After starting this work as a Ph.D. candidate at the University of Calgary in Canada, Khoshooei is now a postdoctoral fellow in Farha’s lab.
Solutions from the closet
The secret behind the new catalyst is molybdenum carbide, an extremely hard ceramic material. Unlike many other catalysts that require expensive metals such as platinum or palladium, molybdenum is a cheap, base metal abundant on Earth.
To convert molybdenum into molybdenum carbide, scientists needed a source of carbon. They found a cheap option in an unexpected place: the closet. Surprisingly, sugar—the white granulated sugar found in almost every household—serves as a cheap and convenient source of carbon atoms.
“Every day when I was trying to synthesize these materials, I brought sugar to the lab from my home,” Khoshooei said. “Compared to other classes of materials commonly used for catalysts, ours is incredibly cheap.”
Successfully selective and stable
When they tested the catalyst, Farha, Khoshway and their collaborators were impressed by its success. Works at ambient pressure and high temperatures (300-600 degrees Celsius), the catalyst converts CO2 in CO with 100% selectivity.
The high selectivity means that the catalyst only acted on CO2 without disturbing the surrounding materials. In other words, industry can apply the catalyst to large volumes of trapped gases and selectively target only CO2. The catalyst also remains stable over time, meaning it remains active and does not degrade.
“In chemistry, it is not uncommon for a catalyst to lose its selectivity after a few hours,” Farha said. “But after 500 hours in harsh conditions, its selectivity has not changed.”
This is particularly remarkable because CO2 is a stable—and persistent—molecule.
“CO Conversion2 it is not easy,” Khoshooei said. “CO2 is a chemically stable molecule, and we had to overcome that stability, which takes a lot of energy.
A tandem approach to carbon cleanup
The development of carbon capture materials is a major focus of Farha’s lab. His group develops metal-organic frameworks (MF), a class of highly porous, nanoscale materials that Farha likens to “sophisticated and programmable bath sponges.” Farha is researching MOFs for various applications, including CO extraction2 directly from the air.
Farha now says that MF and the new catalyst can work together to play a role in carbon capture and sequestration.
“At some point, we could use a MOF to capture CO2, followed by a catalyst converting it into something more useful,” Farha suggested. “A tandem system using two different materials for two consecutive steps could be the way forward.”
“This can help us answer the question, ‘What to do with the captured CO2?'” added Khoshooei. “Right now the plan is to isolate it underground. But underground tanks must meet many requirements to store CO safely and permanently2. We wanted to design a more universal solution that could be used anywhere while adding economic value.”
Reference: “An Active, Stable Cubic Molybdenum Carbide Catalyst for the High-Temperature Reverse Water-Gas Shift Reaction” by Milad Ahmadi Koshoei, Xiyun Wang, Gerardo Vitale, Philip Formalik, Kent O. Kirlikowali, Randall K. Snur, Pedro Pereira-Almao, and Omar K. Farha, 2 May 2024, Science.
DOI: 10.1126/science.adl1260
The study was supported by the US Department of Energy, the National Science Foundation and the Natural Sciences and Engineering Research Council of Canada.