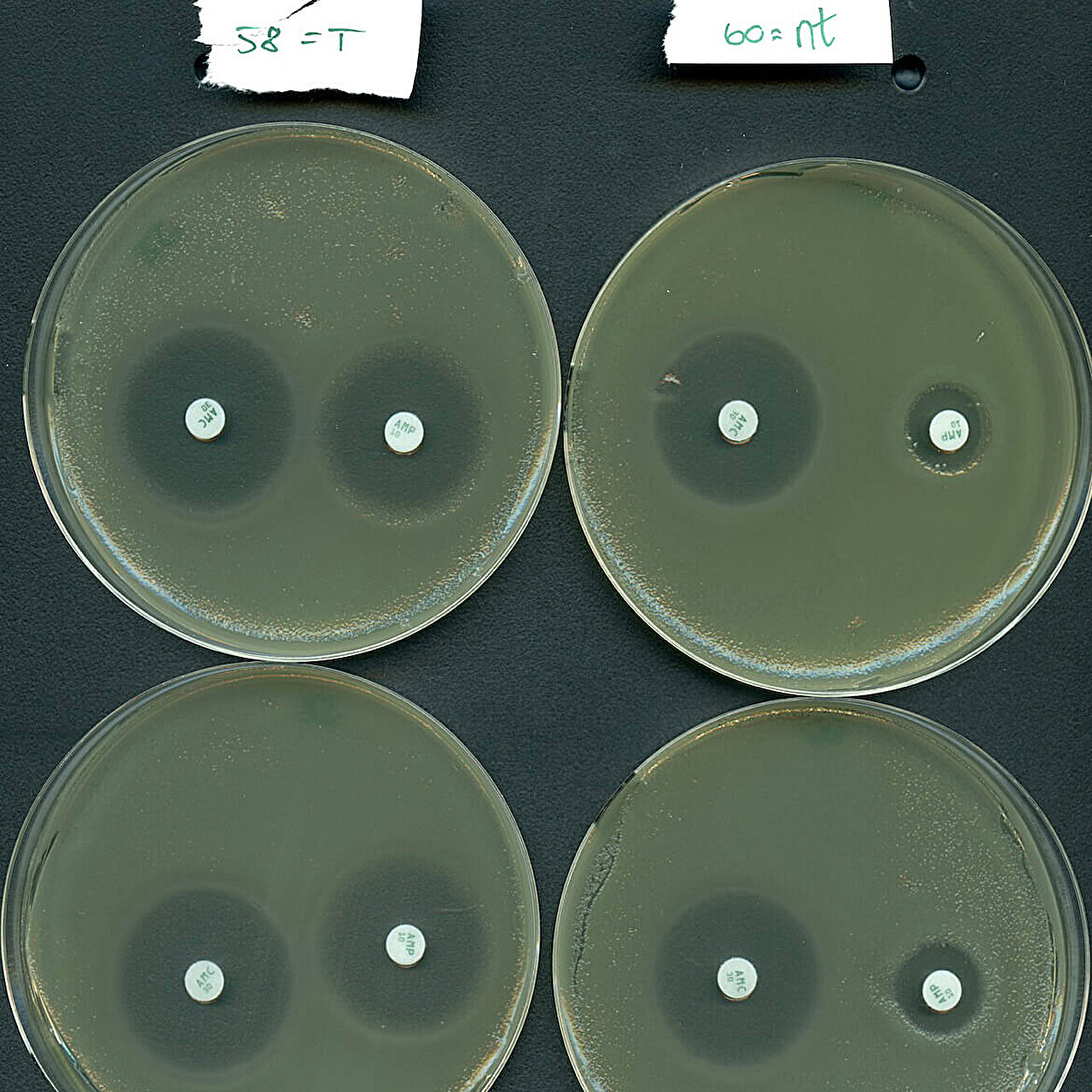
Results of the CRISPR-Cas type IV-A3-mediated antibiotic resistance resensitization assay conducted by the investigators. Credit: Fabien Benz
× near
Results of the CRISPR-Cas type IV-A3-mediated antibiotic resistance resensitization assay conducted by the investigators. Credit: Fabien Benz
CRISPR-Cas systems have revolutionized biotechnology by offering ways to edit genes like a pair of programmable scissors. In nature, bacteria use these systems to fight deadly viruses. A recent international collaboration led by the University of Copenhagen shed light on the most enigmatic CRISPR-Cas systems; the type IV system. Although these atypical systems do not interrupt genes, their unique functions hold promise in our fight against antibiotic resistance.
CRISPR-Cas systems are bacterial adaptive immune systems that target and cut the nucleic acids (DNA/RNA) of invading genetic parasites such as bacteriophages (phages); viruses that infect—and eventually kill—bacterial cells. They consist of two main components; the CRISPR array, which stores the immune memory of past viral infections, and the cas genes (encoding Cas proteins), responsible for coordinating the different stages of the immune response.
There are currently six known types of CRISPR-Cas systems, classified according to their protein composition. All types, except type IV, involve nucleases to cleave DNA/RNA.
CRISPR-Cas systems gained popularity as gene-editing tools, allowing precise programmable cuts at specific genomic locations—eventually leading to the award of the 2020 Nobel Prize in Chemistry for the development of this technology.
Solving the mystery of the missing components
“Type IV systems are the odd cousins among CRISPR-Cas systems because they lack the immune memory acquisition module and the DNA cutting component that made CRISPR-Cas systems so famous. These features and their strict association with mobile circular DNA molecules called plasmids motivated us to take on the task of resolving their intriguing role and their underlying molecular functions,” explains Fabien Benz, postdoctoral researcher at the University of Copenhagen and co-author of a study on this topic published in Cell host and microbe.
Because the hallmark of CRISPR-Cas is their ability to cut DNA at specific locations, type IV systems work in a completely different way. They lack the typical nuclease “scissors” but instead contain DinG helicase, a mysterious protein that unwinds DNA.
“The turning point in this investigation came when we realized that type IV systems do not cut DNA. Instead, we found that they silenced gene expression at their target sites. This is a unique functionality that could have important biotechnological applications,” says Rafael Pinilla-Redondo, assistant professor in the Department of Biology and principal scientific coordinator of the investigation.
The researchers achieved a new breakthrough when they figured out how these systems could function without the necessary components to create immune memory.
“Type IV systems can circumvent the lack of a memory acquisition module by hijacking compatible modules from other CRISPR-Cas systems present in the host bacterium. This is fascinating because these other systems are only distantly related,” explains Sarah Camara-Wilpert, co-first author of this study.
A promising CRISPR tool to fight superbugs
But what is all the fuss about? Well, it turns out that type IV systems have a marked tendency to naturally target plasmids rather than bacterial viruses. Importantly, target plasmids often contain multiple antibiotic resistance genes such as those found in hospital superbugs. Antimicrobial resistance is believed to be directly responsible for over 1 million deaths per year due to treatment failure.
Inspired by their natural plasmid-targeting function, research teams efficiently reprogrammed a type IV system to selectively silence resistance genes carried by high-risk bacteria from hospitalized patients.
“Our results show that type IV systems hold potential as a new tool to combat antibiotic resistance, as we were able to re-sensitize an important pathogen to antibiotic treatment,” says Professor Søren Sørensen, last co-author of the study.
This study was a large interdisciplinary effort involving seven international research groups from different countries. Although the project began as a collaboration between just two groups, it gradually gained momentum, bringing in partners with diverse backgrounds.
“We have experienced a wonderful snowball effect, where each new partner has amplified the impact of the work by sharing their unique skills and providing crucial information to solve the mysteries surrounding Type IV systems.” It was a collaborative tour de force, just how science should be,” notes Pinilla-Redondo.
More info:
Fabienne Benz et al, Type IV-A3 CRISPR-Cas systems drive conflicts between plasmids by acquiring spacers in trans, Cell host and microbe (2024). DOI: 10.1016/j.chom.2024.04.016
Log information:
Cell host and microbe