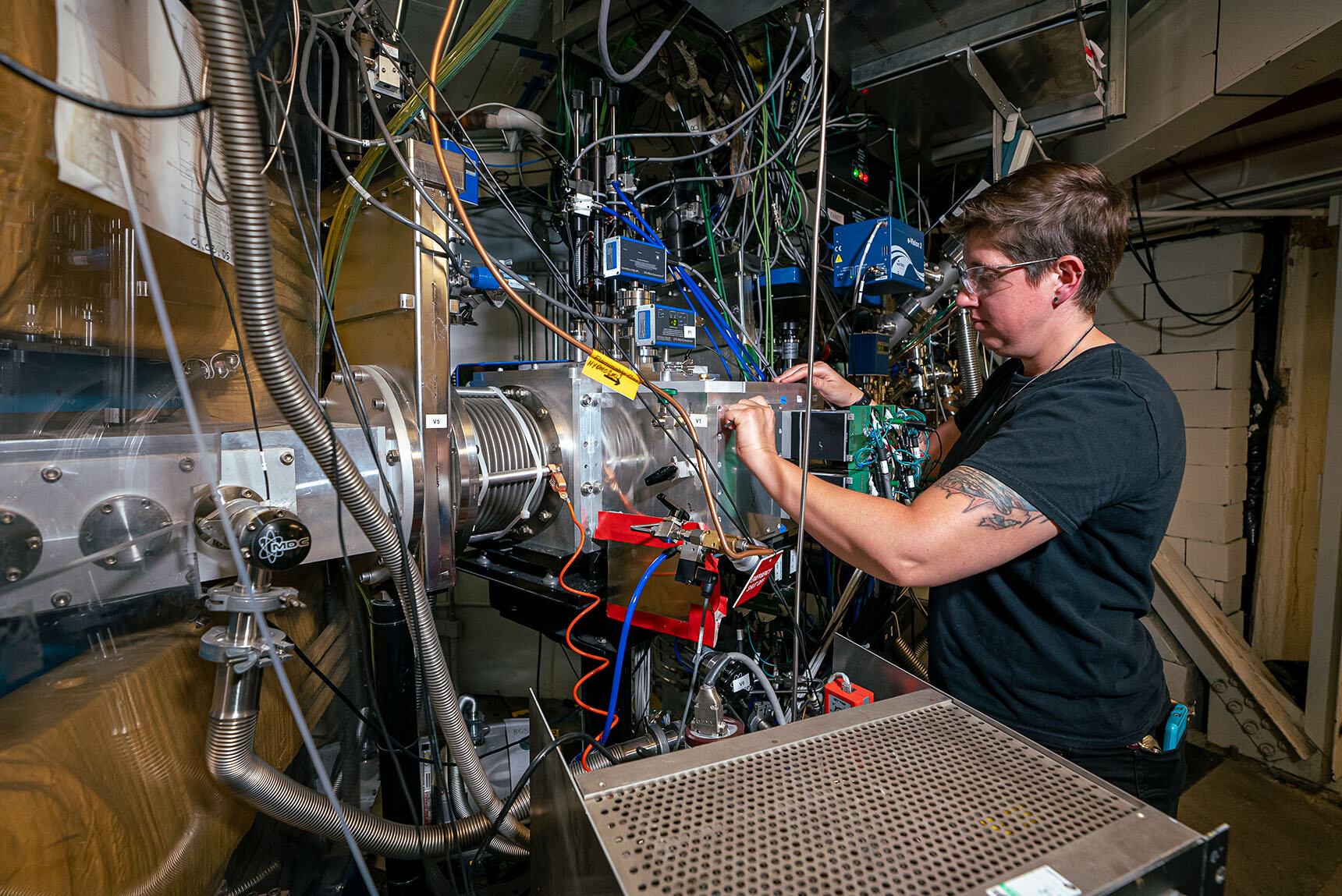
Scientist Jacqueline Gates of the Berkeley gas separator used to split atoms of element 116, livermorium. Credit: Marilyn Sargent/Berkeley Lab
Scientists at the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) are credited with discovering 16 of the 118 known elements. Now they’ve completed the crucial first step to potentially creating one more: element 120.
Today, an international team of researchers led by Berkeley Lab’s Heavy Element Group announced that they have made a known superheavy element 116 using a titanium beam, a breakthrough that is a key step toward creating element 120. The result was presented today at the Nuclear Structure conference 2024; the scientific article will be published in the online repository arXiv and has been submitted to the journal Physical examination letters.
“This reaction had never been demonstrated before, and it was essential to prove it was possible before we embarked on our attempt to make 120,” said Jacqueline Gates, a nuclear scientist at Berkeley Lab leading the effort. “Creating a new item is an extremely rare feat. It’s exciting to be part of the process and have a promising way forward.”
The team made two atoms of element 116, livermorium, during 22 days of operations at the lab’s heavy ion accelerator, the 88-inch cyclotron. Creating an atom of element 120 would be even rarer, but judging by the speed at which they produced 116, it’s a reaction scientists could reasonably be looking for for several years.
“We needed nature to be merciful, and nature was merciful,” said Rainer Kruken, director of Berkeley Lab’s nuclear sciences division. “We think it will take about 10 times longer to do 120 than 116. It’s not easy, but it looks doable now.”
If discovered, element 120 would be the heaviest atom ever created and would occupy the eighth row of the periodic table. It falls on the shores of an “island of stability,” a theorized group of superheavy elements with unique properties.
While the superheavy elements discovered so far decay almost instantly, the right combination of protons and neutrons can create a more stable nucleus that survives longer, giving researchers a better chance to study it. Studying elements at the extremes can provide insight into how atoms behave, test models of nuclear physics, and map the boundaries of atomic nuclei.
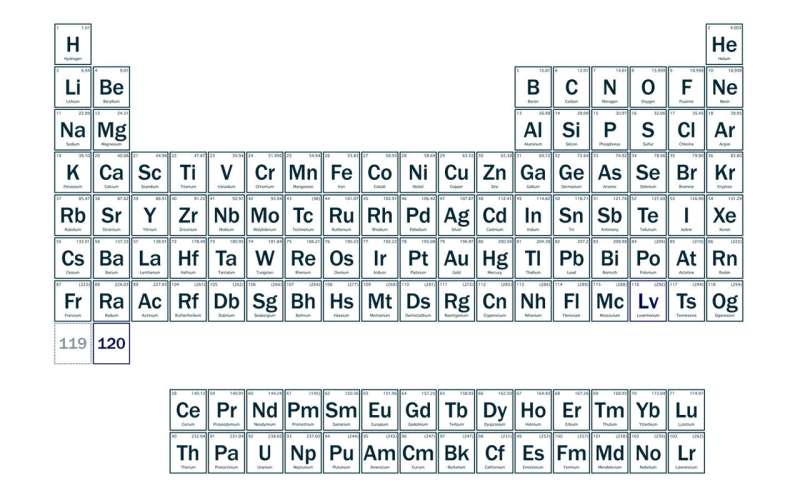
An extended periodic table shows where researchers expect elements 119 and 120 to be categorized if discovered. Credit: Marilyn Sargent/Berkeley Lab
Creation of superheavy elements
The recipe for creating superheavy elements is simple in theory. You smash together two lighter elements that, combined, have the desired number of protons in your final atom. It’s basic math: 1+2=3.
In practice, of course, it is incredibly difficult. It can take trillions of interactions before two atoms successfully fuse, and there are limits to what elements can reasonably be turned into a particle beam or target.
Researchers choose specific isotopes, variants of elements that have the same number of protons but different numbers of neutrons, for their beam and target. The heaviest practical target is an isotope called californium-249, which has 98 protons. (A heavier target, such as one made of a fermium with 100 protons, would decay too quickly). This means that to try to make element 120, researchers can’t use their beam of calcium-48 with its 20 protons. Instead, they need a bundle of atoms with 22 protons: titanium, something not often used in the production of superheavy elements.
The experts at the 88-inch cyclotron set out to see if they could make an intense enough beam of the titanium-50 isotope over a period of weeks and use it to make element 116, the heaviest element ever made at Berkeley Lab.
So far, elements 114 through 118 have only been made with a beam of calcium-48, which has a special or “magic” configuration of neutrons and protons that helps it fuse with target nuclei to produce superheavy elements. The field was open to the question of whether it would even be possible to create superheavy elements near the island of stability using a “non-magical” beam like titanium-50.
“It was an important first step to try to make something a little easier from a new element to see how going from a calcium beam to a titanium beam changes the rate at which we make these elements,” said Jennifer Pore, a scientist in the Heavy Elements Group at Berkeley Lab.
“When we’re trying to make these incredibly rare elements, we’re standing at the absolute edge of human knowledge and understanding, and there’s no guarantee that the physics will work the way we expect.” The creation of element 116 with titanium confirms that this production method works and we can now plan our hunt for element 120.”
The plan to create superheavy elements using Berkeley Lab’s unique facilities is included in the Nuclear Science Advisory Committee’s 2023 Long-Term Nuclear Science Plan.
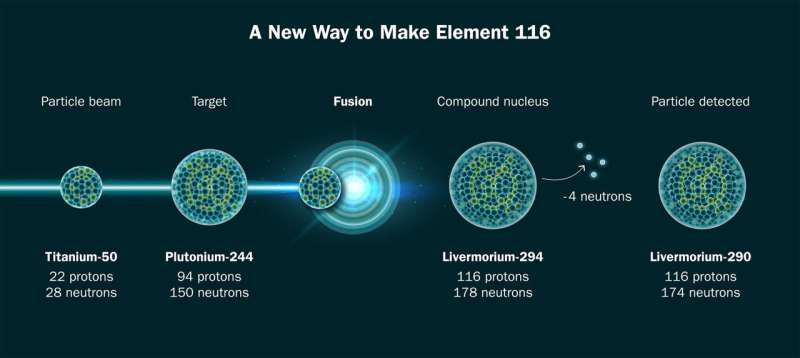
To make element 116, researchers fused isotopes of titanium and plutonium. Credit: Jenny Nuss/Berkeley Lab
Feats of Engineering
Creating a sufficiently intense beam of titanium isotopes is not an easy task. The process starts with a special piece of titanium-50, a rare isotope of titanium that makes up about 5% of all titanium in the earth. This piece of metal goes into an oven about the size of the end segment of your little finger. The furnace heats the metal until it begins to vaporize, similar to the gas released from dry ice, at nearly 3,000 degrees Fahrenheit.
All of this happens inside an ion source called VENUS, a complex superconducting magnet that acts like a bottle sealing the plasma. The free electrons spiral through the plasma, accumulating energy as they are bombarded by microwaves and eject 12 of the 22 electrons on the titanium. Once loaded, the titanium can be maneuvered with magnets and accelerated in the 88-inch cyclotron.
“We knew these high-current titanium beams would be difficult because titanium is reactive with a lot of gases, and that affects the ion source and the stability of the beam,” said Damon Todd, an accelerator physicist at Berkeley Lab and part of the ion team. source. “Our new induction oven can maintain a fixed temperature for days by maintaining a constant output of titanium and directing it precisely at the VENUS plasma to avoid stability issues. We are extremely pleased with our beam production.”
Every second, about 6 trillion titanium ions hit the target (plutonium to make 116, californium to make 120), which is thinner than a sheet of paper and spins to dissipate the heat. Accelerator operators tune the beam to have just the right amount of energy. Too few and the isotopes will not fuse into a heavy element. Too many and the titan will smash the cores into the target.
When the rare superheavy element is formed, it is separated from the rest of the particle debris by magnets in the gas-filled Berkeley Separator (BGS). The BGS transmits it to a sensitive silicon detector known as the SHREC: Super Heavy RECoil detector. SHREC can capture energy, location and time, information that allows researchers to identify the heavy element as it decays into lighter particles.
“We are very confident that we are seeing element 116 and its daughter particles,” Gates said. “There’s about a 1 in 1 trillion chance that this is a statistical fluke.”
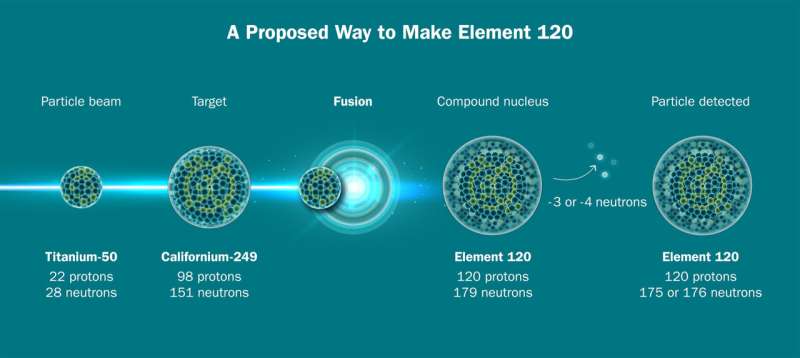
To make element 120, researchers want to fuse isotopes of titanium and californium. Credit: Jenny Nuss/Berkeley Lab
Plans for 120
There is still work to be done before researchers can attempt to make element 120. Experts at the 88-inch cyclotron are still working to prepare the machine for a target made of californium-249, and partners at Oak Ridge National Laboratory will need to make about 45 milligrams of californium in the target.
“We’ve shown that we have a facility capable of doing this project and that the physics seems to make it feasible,” Kruken said. “Once we have our target, shielding and engineering controls in place, we will be ready to take on this challenging experiment.”
The timing has yet to be determined, but researchers could potentially begin the experiment in 2025. Once it begins, it could take several years to see only a few atoms of element 120, if at all.
“We want to understand the limits of the atom and the limits of the periodic table,” Gates said. “The superheavy elements we know so far don’t live long enough to be useful for practical purposes, but we don’t know what the future holds. Maybe it’s a better understanding of how the core works, or maybe it’s something more.”
Collaborations for this work include researchers from Berkeley Lab, Lund University, Argonne National Laboratory, Lawrence Livermore National Laboratory, San José State University, University of Strasbourg, University of Liverpool, Oregon State University, Texas A&M University, UC Berkeley, Oak Ridge National Laboratory , University of Manchester, ETH Zurich and the Paul Scherer Institute.
Courtesy of Lawrence Berkeley National Laboratory
Quote: New way to make element 116 opens door to heavier atoms (2024, July 23), retrieved July 24, 2024, from https://phys.org/news/2024-07-element-door- heavier-atoms.html
This document is subject to copyright. Except for any fair dealing for the purposes of private study or research, no part may be reproduced without written permission. The content is provided for informational purposes only.